Rationale for use in HD, including results from animal studies that indicate efficacy; or compelling evidence from studies in invertebrates or in vitro models suggesting potential activity relevant for HD; or known clinical activity based on previous clinical use.
Compounds For Review
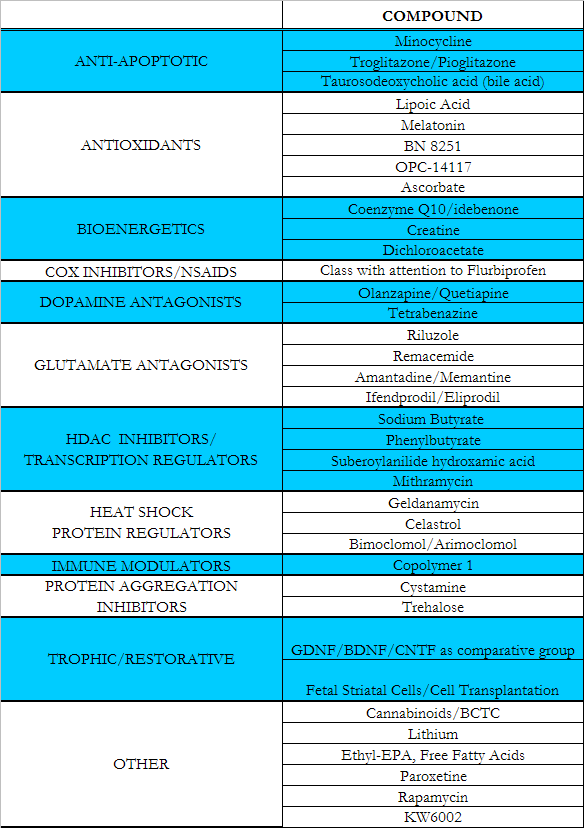
What does “selected for further consideration” mean?
Compounds selected for further consideration will undergo more detail evaluation to find out if they fulfill the operational criteria that were established by the Huntington Project in order to identify compounds likely to make a difference in the lives of people with HD.
What does not being selected mean?
Nothing has been excluded in this search for effective treatments. Many compounds did not make the “short list” only because there was insufficient information available. Other well-known agents have been well described and characterized in other settings and do not need in-depth reviews from SET-HD for the purposes of informing clinical studies. In the future, the working group will reevaluate compounds that were nominated but not selected in this round. Individuals who wish to submit additional information about specific compounds are encouraged to do so on the nomination form.
What compounds have been nominated?
A complete list of compounds nominated in the first round (through March 31, 2004) is available for viewing. You can still NOMINATE A COMPOUND for future consideration.
We invite your comments about these selections and the SET-HD process.
SET-HD Update May 2004
As of March 31, 2004, the SET-HD web site received over 200 nominations of potential interventions for HD. These compounds are now being evaluated by the SET-HD working group in order to identify those with the greatest potential to move rapidly into clinical trials.
The first round of evaluations will focus on agents that have enough safety information to be used in clinical trials in HD in the near future. The intent is to advance the most promising agents whether they are for neuroprotection or symptomatic/ palliative treatment.
All agents will be characterized according to the following criteria: rationale and mechanism, consistency of pre-clinical data, pharmacokinetics and ability to reach the brain in relevant concentrations, and evidence of efficacy in HD animal models. Those people who nominated compounds will be asked to review and comment on the evaluation. Evaluations will be available for review on this web site, and will be published in a peer-reviewed journal.
The first round of evaluations is expected to be complete by 4th quarter of 2004. Subsequent rounds of evaluations will include agents that appear promising because of their particular mechanism of action, even though they may not yet have been tested in humans. The SET-HD working group will identify steps that are needed to develop agents that have not yet made it to human trials.
Compounds may still be nominated through the SET-HD web site. SET-HD is an ongoing process aimed at identifying important treatments for HD.
We will continue to update this site about the progress of SET-HD.
SET- HD Working Group:
NINDS: Bernard Ravina, MD, Jill Heemskerk, PhD, Diane Murphy, PhD, Kenneth Fischbeck, MD, Nicholas DiProspero, MD, PhD, Robert Hart, MD
Academic Investigators: Susan Fagan, PharmD, Collin Hovinga, PharmD, Louis Profenno, MD, PhD, Madeline Harrison, MD
Patient Advocate: James A. Tretheway